Catalytic converters require
Catalytic converters require a temperature of 400 °C (752 °F) to operate effectively.
Therefore, they are place as close to the engine as possible,
or one or more smaller catalytic converters (known as “pre-cats”) are place immediately after the exhaust manifold.
Types of catalytic converters
Two-way A 2-way (or “oxidation”, sometimes called an “oxi-cat”)
catalytic converter has two simultaneous tasks:
Oxidation of carbon monoxide to carbon dioxide: 2 CO + O2 → 2 CO2
Oxidation of hydrocarbons (unburnt and partially burned fuel) to carbon dioxide and water:
CxH2x+2 + [(3x+1)/2] O2 → x CO2 + (x+1) H2O (a combustion reaction)
This type of catalytic converter is widely use on diesel engines to reduce hydrocarbon and carbon monoxide emissions.
They were also use on gasoline engines in American- and Canadian-market automobiles until 1981. Because of their inability to control oxides of nitrogen, they were superse by three-way converters.
Three-way catalytic converters
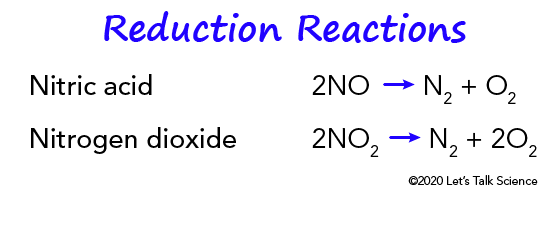
Three-way catalytic converters have the additional advantage of controlling the emission of nitric oxide (NO) and nitrogen dioxide (NO2) (both together abbreviated with NOx and not to be confuse with nitrous oxide (N2O)), which are precursors to acid rain and smog.[19]
Since 1981, “three-way” (oxidation-reduction) catalytic converters have been use in vehicle emission control systems in the United States and Canada;
many other countries have also adopt stringent vehicle emission regulations that in effect require three-way converters on gasoline-powered vehicles.
The reduction and oxidation of catalyst converters
The reduction and oxidation catalysts are typically contained in a common housing; however, in some instances, they may be housed separately. A three-way catalytic converter has three simultaneous tasks:[19]
Reduction of nitrogen oxides to nitrogen (N2)
Oxidation of carbon, hydrocarbons, and carbon monoxide to carbon dioxide
These three reactions occur most efficiently when the catalytic converter receives exhaust from an engine running slightly above the stoichiometric point.
For gasoline combustion, this ratio is between 14.6 and 14.8 parts air to one part fuel, by weight.
The ratio for autogas (or liquefi petroleum gas LPG), natural gas, and ethanol fuels can vary significantly for each, notably so with oxygenated or alcohol based fuels,
with e85 requiring approximately 34% more fuel, requiring modify fuel system tuning and components when using those fuels.
In general, engines fitted with 3-way catalytic converters equipp with a computerize closed-loop feedback fuel injection system using one or more oxygen sensors,[citation need]
though early in the deployment of three-way converters, carburetors equipped with feedback mixture control were used.
Three-way converters are effective when the engine is operate within a narrow band of air-fuel ratios near the stoichiometric point.[20]
Total conversion efficiency falls very rapidly when the engine is operate outside of this band. Slightly lean of stoichiometric,
the exhaust gases from the engine contain excess oxygen, the production of NOx by the engine increases, and the efficiency of the catalyst at reducing NOx falls off rapidly.
However, the conversion of HC and CO is very efficient due to the available oxygen, oxidizing to H2O and CO2. Slightly rich of stoichiometric, the production of CO and unburned HC by the engine starts to increase dramatically,
available oxygen decreases, and the efficiency of the catalyst for oxidizing CO and HC decreases significantly, especially as stored oxygen becomes depleted.
However, the efficiency of the catalyst at reducing NOx is good, and the production of NOx by the engine decreases. To maintain catalyst efficiency, the air:fuel ratio must stay close to stoichiometric and not remain rich or lean for too long.
effective operation of three-way catalytic converters
Closed-loop engine control systems are used for effective operation of three-way catalytic converters because of this continuous rich-lean balance required for effective NOx reduction and HC+CO oxidation.
The control system allows the catalyst to release oxygen during slightly rich operating conditions, which oxidizes CO and HC under conditions that also favor the reduction of NOx.
Before the stored oxygen is deplet, the control system shifts the air:fuel ratio to become slightly lean, improving HC and CO oxidation while storing additional oxygen in the catalyst material, at a small penalty in NOx reduction efficiency.
Then the air:fuel mixture is brought back to slightly rich, at a small penalty in CO and HC oxidation efficiency, and the cycle repeats. Efficiency is improved when this oscillation around the stoichiometric point is small and carefully controlled.[21]
Closed-loop control under light to moderate load is accomplished by using one or more oxygen sensors in the exhaust system. When oxygen is detected by the sensor, the air:fuel ratio is lean of stoichiometric, and when oxygen is not detected, it is rich.
The control system adjusts the rate of fuel being injected into the engine based on this signal to keep the air:fuel ratio near the stoichiometric point in order to maximize the catalyst conversion efficiency.
The control algorithm is also affect by the time delay between the adjustment of the fuel flow rate and the sensing of the chang air:fuel ratio by the sensor, as well as the sigmoidal response of the oxygen sensors.
Typical control systems are design to rapidly sweep the air:fuel ratio such that it oscillates slightly around the stoichiometric point, staying near the optimal efficiency point while managing the levels of stored oxygen and unburnt HC.[20]
Closed loop control of catalytic converters
Closed loop control is often not used during high load/maximum power operation, when an increase in emissions is permitted and a rich mixture is commanded to increase power and prevent exhaust gas temperature from exceeding design limits.
This presents a challenge for control system and catalyst design.
During such operations, large amounts of unburnt HC are produce by the engine, well beyond the capacity of the catalyst to release oxygen.
The surface of the catalyst quickly becomes saturated with HC. When returning to lower power output and leaner air:fuel ratios,
the control system must prevent excessive oxygen from reaching the catalyst too quickly, as this will rapidly burn the HC in the already hot catalyst, potentially exceeding the design temperature limit of the catalyst.
Excessive catalyst temperature can prematurely age the catalyst, reducing its efficiency before reaching its design lifetime.
Excessive catalyst temperature can also be cause by cylinder misfire, which continuously flows unburnt HC combined with oxygen to the hot catalyst, burning in the catalyst and increasing its temperature.[22]
Unwanted reactions of catalytic converters
Unwanted reactions result in the formation of hydrogen sulfide and ammonia, which poison catalysts.
Nickel or manganese is sometimes add to the washcoat to limit hydrogen-sulfide emissions.[citation needed] Sulfur-free or low-sulfur fuels eliminate or minimize problems with hydrogen sulfide.
Diesel engines of catalytic converters
For compression-ignition (i.e., diesel) engines, the most commonly used catalytic converter is the diesel oxidation catalyst (DOC). DOCs contain palladium and/or platinum supported on alumina.
This catalyst converts particulate matter (PM), hydrocarbons, and carbon monoxide to carbon dioxide and water.
These converters often operate at 90 percent efficiency, virtually eliminating diesel odor and helping reduce visible particulates.
These catalysts are ineffective for NOx, so NOx emissions from diesel engines are controlled by exhaust gas recirculation (EGR).
In 2010, most light-duty diesel manufacturers in the U.S. added catalytic systems to their vehicles to meet federal emissions requirements.
Two techniques have been developed for the catalytic reduction of NOx emissions under lean exhaust conditions, selective catalytic reduction (SCR) and the NOx adsorber.
Instead of precious metal-containing NOx absorbers, most manufacturers select base-metal SCR systems that use a reagent such as ammonia to reduce the NOx into nitrogen and water.[23]
Ammonia is supply to the catalyst system by the injection of urea into the exhaust, which then undergoes thermal decomposition and hydrolysis into ammonia. The urea solution is also refer to as diesel exhaust fluid (DEF).
Diesel exhaust contains relatively high levels of particulate matter.
Catalytic converters remove only 20–40% of PM so particulates are cleaned up by a soot trap or diesel particulate filter (DPF).
In the U.S., all on-road light, medium, and heavy-duty diesel-power vehicles built after 1 January 2007, are subject to diesel particulate emission limits, and so are equipp with a 2-way catalytic converter and a diesel particulate filter.[citation need]
As long as the engine was manufactured before 1 January 2007, the vehicle is not require to have the DPF system.[citation needed] This led to an inventory runup by engine manufacturers in late 2006 so they could continue selling pre-DPF vehicles well into 2007.[24]
Lean-burn spark-ignition engines
For lean-burn spark-ignition engines, an oxidation catalyst is use in the same manner as in a diesel engine. Emissions from lean burn spark ignition engines are very similar to emissions from a diesel compression ignition engine.
Installation of catalytic converters
Many vehicles have a close-couple catalytic converter located near the engine’s exhaust manifold. The converter heats up quickly, due to its exposure to the very hot exhaust gases,
enabling it to reduce undesirable emissions during the engine warm-up period. This is achieve by burning off the excess hydrocarbons which result from the extra-rich mixture require for a cold start.
When catalytic converters were first introduced, most vehicles used carburetors that provided a relatively rich air-fuel ratio.
Oxygen (O2) levels in the exhaust stream were therefore generally insufficient for the catalytic reaction to occur efficiently. Most designs of the time therefore included secondary air injection, which injected air into the exhaust stream. This increased the available oxygen, allowing the catalyst to function as intended.
Some three-way catalytic converter systems
As in two-way converters, this injected air provides oxygen for the oxidation reactions.
This causes unburned fuel to ignite in the exhaust tract, thereby preventing it reaching the catalytic converter at all. This technique reduces the engine runtime needed for the catalytic converter to reach its “light-off” or operating temperature.
Most newer vehicles have electronic fuel injection systems, and do not require air injection systems in their exhausts. Instead, they provide a precisely controlled air-fuel mixture that quickly and continually cycles between lean and rich combustion.
Oxygen sensors monitor the exhaust oxygen content before and after the catalytic converter,








 catalytic converters
catalytic converters



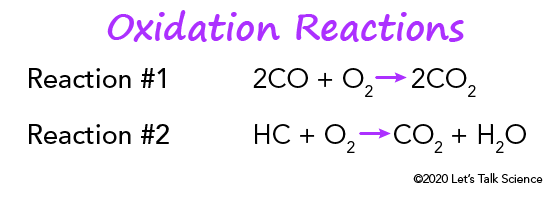 Oxidation reactions for carbon monoxide and unburned hydrocarbons (© 2019 Let’s Talk Science).Modern catalytic converters also use oxygen sensors. They’re sometimes called . They control how much extra oxygen gets pumped into the exhaust stream.
Oxidation reactions for carbon monoxide and unburned hydrocarbons (© 2019 Let’s Talk Science).Modern catalytic converters also use oxygen sensors. They’re sometimes called . They control how much extra oxygen gets pumped into the exhaust stream.
